AACC 2008 - Clinical Lab Expo
Washington DC, USA
July 29-31, 2008 Booth no. 1561 For the first time, Liofilchem® exhibited at the international trade fair AACC - Clinical Lab Expo in the United States. |
|
 April 3, 2008
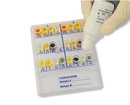
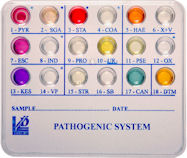
New PATHOGENIC SYSTEM
We are glad to announce the new PATHOGENIC SYSTEM (ref. 71679), a 18 wells system containing biochemical substrata for the detection and presumptive identification of pathogenic microorganisms from pharyngeal, cutaneous, auricular and ophthalmic swabs, expectorate, exudate from abscessand injury, and other clinical specimens in only 18-24 hours.
The system is inoculated directly with the suspension of the clinical specimen and incubated at 36 °C.
The tests for the detection and identification of microorganisms present in the specimen are interpreted by evaluating the color change in the wells and performing immunologic and microscopic confirmation tests.
The new PATHOGENIC SYSTEM allows detection and presumptive identification of Streptococcus pyogenes (Group A), Staphylococcus aureus, Haemophylus spp., Escherichia coli, Proteus spp./Providencia spp., Pseudomonas spp., KES group microorganisms, Enterococcus faecalis, Candida spp., and dermatophytes of the genus Microsporum spp., Trichophyton spp., Epidermophyton spp..
The new PATHOGENIC SYSTEM is already available for sale and samples.
|
|
 February 25, 2008
Chromatic: new Liofilchem® chromogenic culture media
We are glad to introduce our new chromogenic culture media into the Liofilchem® product catalogue 2008, called Chromatic, available in powder and ready to use in petri dish or bottle.
Our new chromogenic culture media are:
• Chromatic Candida • Chromatic Coli/Coliform • Chromatic Salmonella • Chromatic Detection The previous versions (Colorex Candida, Colorex Coli/Coliform, Colorex Salmonella, Colorex Detection) are no more available in the Liofilchem® catalogue.
The new Chromatic media provide optimum results, in terms of effectiveness, qualitative and colorimetric performance. | |
 January 14, 2008
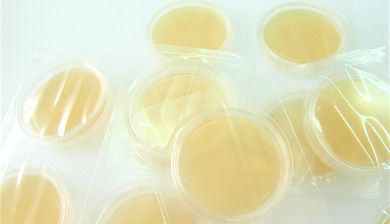
Contact plates (Rodac) in peelable blister pack
We announce with great satisfaction the new peelable blister pack for our contact plates.
The new peelable blister pack fulfills the highest quality and protection standards for the plate. The blister pack, produced fully automatically and in controlled environment, offers the following advantages compared to the pouch pack: - full transparency of the package; - does not release particles during the film opening phase, critical in environment controlled by particle counter; - allows the usage of single plates, leaving the remaining plates protected in their pack; - drastically reduces the dehydration of culture medium during the shelf life period; - permits better product preservation and isolation during transportation and storage phases; - guarantees the product can not be handled after the packaging; - enables the immediate individuation of possible culture medium alteration previous to opening the package, therefore avoiding contaminations to the controlled environment. Price and ref. of the contact plates remain unvaried. The contact plates in the new peelable blister pack are already available. | |
January 9, 2008
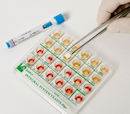
New COPRO SYSTEM Plus
We are glad to announce the evolution of our microbial identification system COPRO SYSTEM from fecal specimens.
The new COPRO SYSTEM Plus (ref. 71675) is a 18 wells system containing dried biochemical substrata for detection and presumptive identification of microorganisms in fecal specimens.
COPRO SYSTEM Plus allows detection and presumptive identification within 18-24 hours of Salmonella spp., Proteus spp., Pseudomonas spp., Yersinia spp., Shigella spp., Campylobacter jejunii, Escherichia coli, E.coli O157 enteropathogenic, KES group microorganisms (Klebsiella, Enterobacter, Serratia), Candida spp.
The system is inoculated directly with the suspension of the specimen under examination and incubated at 36 ± 1 °C.
The tests for detection and presumptive identification of the microorganisms present in the specimen are interpreted evaluating:
• color change in the well • immuno-serological confirmation test • performing a microscopical examination. The COPRO SYSTEM Plus will be available from February 1, 2008.
The COPRO SYSTEM (ref. 71670) remains available in the Liofilchem® product catalogue. |
Copyright © 2024 Liofilchem® srl - All Rights Reserved